By- Christina Sandra Singh
The FDA has issued a second procedural notice of 30 days to conduct quantitative research on front-of-package labelling of packaged food products. Front-of-Package (FOP) labels highlight important nutrients and help consumers make informed decisions about food purchases.
The main goal of this study is to further analyse the responses of consumers and their thoughts about various schemes for front-of-package labelling. Also, this study can assist in exploring the development of these labelling schemes. On top of that, according to the Paperwork Reduction Act, it’s necessary for the FDA and other federal agencies to publish a notice of each and every proposed and collected piece of information in the federal register, which can provide consumers with the opportunity to comment.
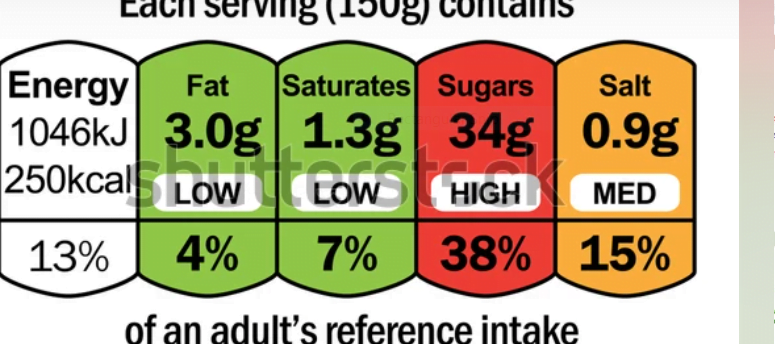
The intention behind this front-of-package labelling is to complement the nutrition facts label on packaged food items. It provides additional contents to consumers and helps them know and choose healthier food alternatives. Especially those with less knowledge about proper nutrition and healthy choices, can benefit from these standardized schemes and adopt a safe and healthier eating pattern. Moreover, the countries that adopted these schemes gave positive feedback that these labelings can assist in more nutrition-based awareness, healthier eating choices, and nutrition comprehension.
The FDA continues to promote nutritional activities that can aid consumers in identifying and making the right choice for their food and beverages through nutritional information on the food and drink labels. The reason behind the agency’s continuous focus on nutritional activities is the increasing number of chronic diseases cases related to improper diet in the US.
For consumers, from July 15, 2023, the comment period opens where they can bring forth their opinion about this matter. Also, by July 17, 2023, comments on the notice are due to OMB. To find this information, consumers can visit Regulations.gov and select “Currently Under Review—Open for Public Comments.” Consumers can also check the Federal Register Notice to comment or see additional related information.
While front-of-package labelling may need an overhaul to better serve consumers, the current method has been shown to be effective in one respect. A 2020 Journal of Marketing study that looked at 21,096 products using Facts Up Front found a relationship between front-of-pack labelings and better nutritional quality.
This isn’t the only front-of-packaging scheme the FDA is looking into right now. The agency has also been studying the effectiveness of a “healthy” symbol, which could tell consumers at a glance whether the product meets the FDA’s definition of the term.